Watson and Crick immediately saw the relationship of the double helix to genetic replication. They proposed that each strand of the chromosome serves as a
template to specify a new, complementary DNA strand. A template is a pattern for making something; DNA acts as a template because each strand specifies the new
daughter strand by base-pairing. This template feature makes DNA replication
semiconservative: after replication, each
daughter chromosome has one strand of newly synthesized DNA and one strand of DNA from the parental chromosome. See Figure
1 .
All DNA polymerases require a template strand, which is copied. DNA polymerases also require a primer, which is complementary to the template. The reaction of DNA polymerases is thus better understood as the addition of nucleotides to a primer to make a sequence complementary to a template. The requirement for template and primer are exactly what would be expected of a replication enzyme. Because DNA is the information store of the cell, any ability of DNA polymerases to make DNA sequences from nothing would lead to the degradation of the cell's information copy.
More than one DNA polymerase exists in each cell. The key distinction among the enzyme forms is their processitivity—how long a chain they synthesize before falling off the template. A DNA polymerase used in replication is more processive than a repair enzyme. The replication enzyme needs to make a long enough chain to replicate the entire chromosome. The repair enzyme needs only to make a long enough strand to replace the damaged sequences in the chromosome. The best-studied bacterium, E. coli, has three DNA polymerase types.
DNA polymerase I (Pol I) is primarily a repair enzyme, although it also has a function in replication. About 400 Pol I molecules exist in a single bacterium. DNA polymerase I only makes an average of 20 phosphodiester bonds before dissociating from the template. These properties make good sense for an enzyme that is going to replace damaged DNA. Damage occurs at separate locations so the large number of Pol I molecules means that a repair enzyme is always close at hand.
The actual replication enzyme in E. coli is
DNA polymerase III. Its properties contrast with Pol I and Pol II in several respects. Pol III is much more processive than the other enzymes, making about 500,000 phosphodiester bonds on the average. In other words, it is about 5,000 times more processive than Pol I and 50 times more processive than Pol II. Pol III is a multisubunit enzyme. It lacks a 5′ to 3′ exonucleolytic activity, although a subunit of the enzyme carries out the editing (3′ to 5′) function during replication. Finally, only about 10 molecules of Pol III reside in each cell. This remains consistent with the function of Pol III in replication, because the chromosome only needs to be copied once per generation. Therefore, the cell only requires a few molecules of the enzyme. Pol III synthesizes DNA at least a hundred times more rapidly than the other polymerases. It can synthesize half of the bacterial chromosome in a little more than 20 minutes, which is the fastest that the bacterium can replicate.
Elongation
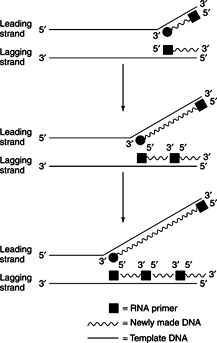
Because only one strand can serve as a template for synthesis in the 5′ to 3′ direction (the template goes in the 3′ to 5′ direction, because the double helix is antiparallel), only one strand, the leading strand, can be elongated continuously. Ahead of the replication fork, DNA gyrase (topoisomerase II) helps unwind the DNA double helix and keep the double strands from tangling during replication.
Synthesis of the second (lagging) strand is more complicated because it is going in the wrong direction to serve as a template. No DNA polymerase exists to synthesize DNA in the 3′ to 5′ direction, so copying of the lagging strand isdiscontinuous—that is, short strands of DNA are made and subsequently matured by joining them together. An RNA primer, which is made by primase, initiates each of these small pieces of DNA. Then DNA polymerase III elongates the primer until it butts up against the 5′ end of the next primer molecule.
DNA polymerase I then uses its polymerizing and 5′ to 3′ exonuclease activities to remove the RNA primer and fill in this sequence with DNA. Because Pol I is not very processive, it falls off the lagging strand after a relatively short-length synthesis. DNA polymerases can't seal up the nicks that result from the replacement of RNA primers with DNA. Instead, another enzyme, DNA ligase, seals off the nicks by using high energy phosphodiester bonds in ATP or NAD to join a free 3′ hydroxyl with an adjacent 5′ phosphate.







0 comments:
Post a Comment